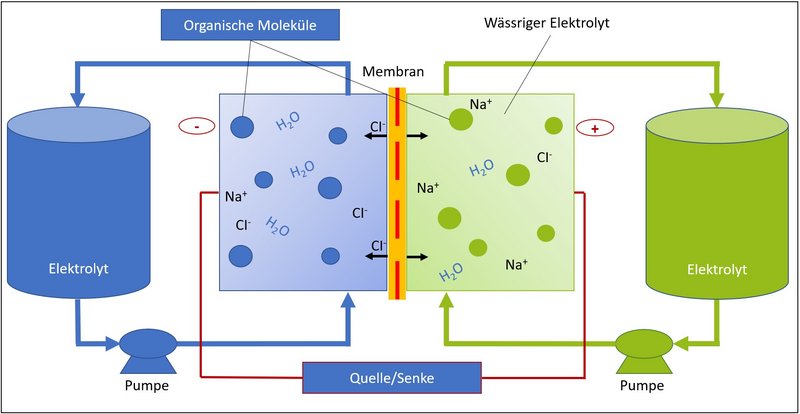
What is a redox flow battery?
Redox flow batteries, also called redox flow battery, flow battery or liquid battery, provide electrical energy from liquid electrolyte solutions, often based on the heavy metal vanadium. The difference to the rechargeable battery (also called accumulator) is the spatial separation between the two stores of the redox flow battery, each containing electrolyte liquids of different concentrations, and the energy converter, whose cells consist of a membrane and two electrodes.
These cells process the electrolyte liquid in a chemical reaction that provides usable electrical energy. This reaction is reversible, so that with the help of electrical energy, for example from renewable sources, the electrolyte fluid returns to its original initial concentration and can thus provide electrical energy again in the next step. A sustainable cycle is created that can be used to store electricity.